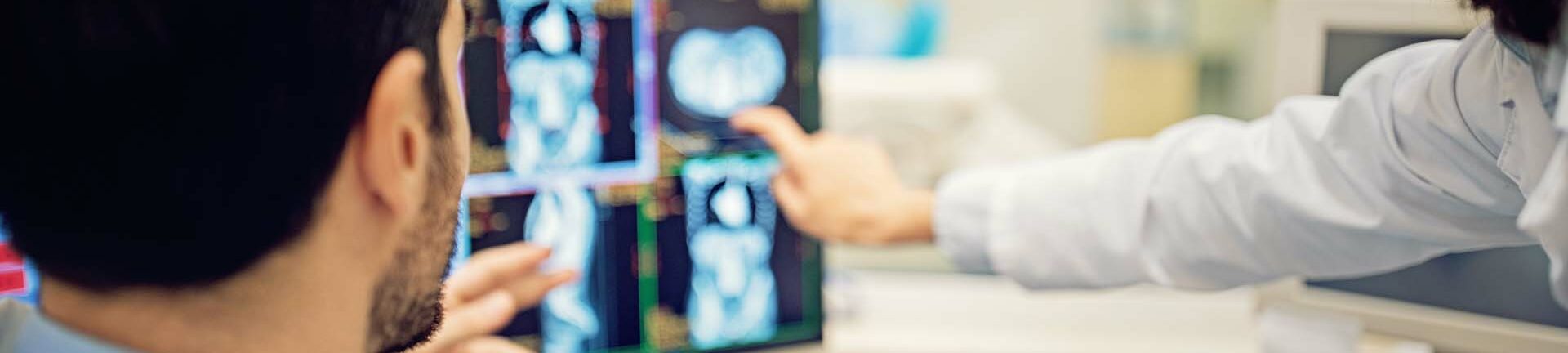
Safety & Vigilance
We provide comprehensive pharmacovigilance and medical device vigilance services from the early stage of development, all the way through to the marketing of your medicinal products and medical devices.
Our highly-trained team in regulatory pharmacovigilance, including a European Qualified Person for Pharmacovigilance (EU-QPPV), are supported by our clinical experts, with our physicians covering a wide range of medical specialties.
How can we help you?
Contact our expert team to discover how Astrum can bring value to your clinical development projects.
Other services
Regulatory Affairs
We provide comprehensive regulatory services to help you navigate the ever-changing regulatory landscape and ensure efficient delivery of your clinical studies
Medical Monitoring
Acting as a point of reference for study team members and investigative sites, our Medical Monitors ensure the safety and integrity of the subjects throughout the trial
Clinical Development & Scientific Advice
Ranging from first in human to post-marketing, Astrum’s clinical development expertise spans the entire development cycle, offering support every step of the way