Phase I & Clinical Pharmacology
Phase I & Clinical Pharmacology
One of Europe's Most Accomplished
Hospital-Based Phase I Units
Astrum’s early development capabilities are anchored in Porto, Portugal – home to Astrum’s own hospital-based Phase I facility. With 60 dedicated beds and direct access to hospital services, our Clinical Pharmacology Unit (CPU) delivers the speed, safety, and scientific rigor required for First-in-human and early-stage studies.

Located within a hospital environment, the CPU ensures:
Immediate emergency and ICU support
On-site laboratory and imaging facilities
Logistical efficiency and a secure setting for study participants
Capability for complex and high-risk study designs
Capabilities Overview
At Astrum’s Porto Phase I Unit, we deliver a comprehensive suite of clinical pharmacology studies, combining the safety of a hospital-based environment with the efficiency of a dedicated research facility.
Our capabilities cover the full range of study types required for regulatory submission, investor confidence, and early proof-of-concept.
First-in-Human (FIH) & SAD/MAD Studies
Pharmacokinetics & Pharmacodynamics (PK/PD)
Bioavailability &
Bioequivalence
DDIs
Special
Populations
Additional Capabilities:
- Cardiac Safety & Thorough QT (TQT) Evaluations
- Proof-of-Concept (POC) Studies in Patients
- CSF Collection & Analysis

Across Astrum
- Drug–Device Combination Studies
- Irritation, Sensitization & Adhesion Studies
- Specialty Procedures (biopsies, NG tube, unique administration routes)
Astrum Phase I Unit - Phase I Excellence
First-in-Human
SAD / MAD
BA / BE
Drug—Drug & Food Effect
PK / PD
AME / Metabolite Profiling
Cardiac Safety
Drug—Device Studies
Irritation / Sensitization
Astrum Porto
Phase I Unit
60 Beds I EMA FDA, ANVISA Inspected
Renal Impairment
Hepatic Impairment
Smokers
Obese Patients
Women's Health
GLP-I Patients
POC Studies
CSF Collection Feasibility
Specialty Procedures
Astrum Porto’s hospital-based Phase I Unit delivers the full spectrum of clinical pharmacology studies. With 60 beds and Inspections by EMA, FDA, and ANVISA, amongst others, Sponsors gain confidence in both scientific rigor and global regulatory acceptance.
to see how Astrum Porto supports early development from First-in-Human and Proof-of-Concept.
Access to Special Populations
Through our hospital network in Portugal, we provide direct access to patient groups critical for translational medicine:

CSF Collection & Analysis
neurological and CNS studies

GLP-1 Treated Patients
increasingly relevant in obesity and diabetes programs

Hepatic Impairment
full spectrum of liver dysfunction

Obese Patients
cardiometabolic and endocrinology programs

Proof-of-Concept Studies in Patients
seamless transition from healthy volunteers

Renal Impairment
mild, moderate, severe, and dialyzed

Smokers
controlled cohorts for metabolic and respiratory studies

Specialty Procedures
biopsies and unique routes of administration

Women’s Health
reproductive, gynecology, hormonal therapy
Our Dedicated Phase I Experts
Behind the Porto Phase I Unit is a in-house team of seasoned clinical investigators and medical specialists dedicated to advancing early development. With decades of combined experience in first-in-human studies, DDI, and regulatory scrutiny, our leadership ensures:
Participant safety through hospital-based oversight
Scientific rigor in study design and execution
Regulatory confidence with EMA, FDA, and ANVISA-inspected operations

Cristina Lopes
Chief Operating Officer
Portugal

Cristina Lopes
Chief Operating Officer
Cristina Lopes has extensive experience in patient recruitment and site management, with a focus on Phase I trials and early patient engagement strategies. She has developed site networks and processes that improved trial efficiency and participant retention across Europe. At Astrum, Cristina leads site and patient services, ensuring trials recruit effectively and deliver meaningful results.

Prof. Francisco Pimentel
Chief Medical Officer
Portugal

Prof. Francisco Pimentel
Chief Medical Officer
Prof. Francisco Pimentel is a senior leader with over 40 years of experience in Medicine and Clinical Research. He holds an MD and a PhD, and specializes in Oncology, Internal Medicine, Clinical Pharmacology, and Pharmaceutical Medicine. His expertise spans all clinical phases (I-IV), including extensive PI experience in Oncology (60+ trials). As Senior Director, he oversees all core medical, scientific, regulatory, and safety services, providing strategic guidance and medical safety oversight across all development programs.

Dr. Marlene Fonseca
MD Phase I Clinical Director
Portugal

Dr. Marlene Fonseca
MD Phase I Clinical Director
Dr. Marlene Fonseca is the Phase I Clinical Director, specializing in Cardiology and Clinical Pharmacology. Her dual board certification and Advanced Life Support (ALS) certification provide exceptional safety oversight for early-phase trials. As a Principal Investigator, she has led over 50 Phase I trials among 150+ trials total. Her expertise encompasses all trial types – from generics and innovative drugs (First-in-Human, SAD, MAD) to those with stringent safety profiles and specialized cardiac assessments like Thorough QT (TQT) studies.

Ricardo Cunha
Manager of Scientific Affairs
Portugal

Ricardo Cunha
Manager of Scientific Affairs
Ricardo Cunha is a scientific and regulatory strategist with over 12 years of experience in the R&D field, holding a PharmD and an MSc in Pharmaceutical Medicine. He specializes in supporting sponsors by establishing compliant non-clinical and clinical development plans and study designs, both for innovative and generic medicines. Ricardo ensures full regulatory compliance with worldwide authorities, including the US FDA, EMA, and ANVISA. He leads the preparation of scientific and regulatory advice packages to streamline proposals, minimizing overall investment and time to market.

Manuel Azevedo
Director of Phase I Operations
Portugal

Manuel Azevedo
Director of Phase I Operations
Manuel Azevedo is a seasoned clinical leader with over two decades in Phase I research (200+ trials). Currently serving as Director of Phase I Operations, he leads all Phase I operational departments, overseeing the comprehensive planning, preparation, and execution of clinical studies. His leadership is underpinned by extensive experience in Emergency Medicine and his Advanced Life Support certification, ensuring superior clinical management and participants’ safety. He is responsible for overall operational readiness, budget control, and strict adherence to global quality standards.

Prof. Serafim Guimarães
Specialist in Nephrology and Clinical Pharmacology
Portugal

Prof. Serafim Guimarães
Specialist in Nephrology and Clinical Pharmacology
Prof. Serafim Guimarães is a dual board-certified specialist in Nephrology and Clinical Pharmacology, also Academic Associate Professor, with over 20 years in clinical research. Serving as Principal Investigator (PI) in over 90 Phase I trials, he specializes in overseeing complex bioequivalence (BE) and special population studies, notably renal impairment trials including hemodialysis cohorts. His high standards for quality are proven by the research projects he has overseen collecting multiple No Action Indicated (NAI) classifications from the US FDA.
Behind the Porto Phase I Unit is a in-house team of seasoned clinical investigators and medical specialists dedicated to advancing early development. With decades of combined experience in first-in-human studies, DDI, and regulatory scrutiny, our leadership ensures:
Participant safety through hospital-based oversight
Scientific rigor in study design and execution
Regulatory confidence with EMA, FDA, and ANVISA-inspected operations

Dr. Cristina Lopes
Medical Director
With over 20 years in clinical research, Dr. Vance is a leader in early-phase trial design and patient safety protocols.

Dr. Marlene Fonseca
Principal Investigator
Dr. Thorne specializes in first-in-human studies and has successfully led over 50 Phase I trials to completion.

Dr. Francisco Pimentel
Clinical Pharmacology Lead
An expert in pharmacokinetics and pharmacodynamics, Dr. Reyes ensures scientific integrity in every study protocol.
Access to Special Populations
Through our hospital network in Portugal, we provide direct access to
patient groups critical for translational medicine:
Renal Impairment
mild, moderate, severe, and dialyzed
Hepatic Impairment
full spectrum of liver dysfunction

Smokers
controlled cohorts for metabolic and respiratory studies

Obese Patients
cardiometabolic and endocrinology programs

Women’s Health
reproductive, gynecology, hormonal therapy
GLP-1 Treated Patients
increasingly relevant in obesity and diabetes programs

Proof-of-Concept Studies in Patients
seamless transition from healthy volunteers

CSF Collection & Analysis
neurological and CNS studies

Specialty Procedures
biopsies and unique routes of administration
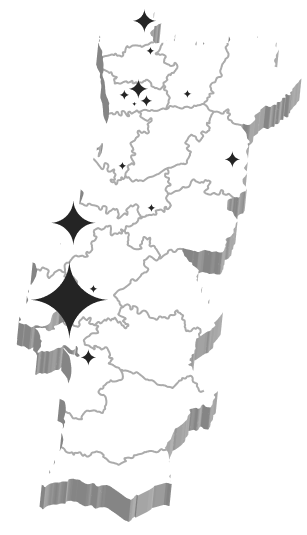
Clinical Research Network
Astrum is managing a clinical research network in Portugal through established partnerships with eleven institutions belonging to the National Healthcare System.
- Over 400 clinical studies in patients being managed through this network
- Seamless access to diverse patient populations, including renal and hepatic impairment, smokers, obese patients, and women’s health
- Ability to conduct both healthy volunteer and patient studies within an integrated framework
This hospital network ensures that Astrum can support not only early pharmacology studies, but also proof-of-concept trials in patients, accelerating the path from first-in-human to later clinical phases.


Our Location – Porto, Portugal
Astrum’s Clinical Pharmacology Unit is located within Hospital da Prelada, ensuring immediate hospital support, laboratory access,
and emergency care capabilities.
Hospital da Prelada
Rua de Sarmento de Beires, 153
4250-449 Porto, Portugal
+351 220 959 020
Astrum’s Porto Phase I Unit combines the security of a hospital-based environment with the efficiency of a dedicated clinical pharmacology facility. Managed by our own clinical team.
Full Suite of Clinical Pharmacology Capabilities
We provide end-to-end expertise across the full spectrum of early development studies
Access to Special Populations
Launching an early-phase trial requires speed as much as scientific rigor. Portugal is recognized as one of the fastest approval environments in Europe for Phase I and first-in-human studies.
Streamlined process
Regulatory review is conducted by INFARMED with a centralized ethics committee, avoiding delays linked to multiple local reviews.
Accelerated timelines
Phase I studies in Portugal can often be approved in 6 weeks on average (31 days).
Predictable interactions
INFARMED has a strong reputation for responsiveness, clarity, and consistent guidance.
Global credibility
Approvals and inspections in Portugal are fully aligned with EMA, FDA, and ANVISA requirements, ensuring international acceptance.
This regulatory efficiency, combined with Astrum’s hospital-based infrastructure in Porto, means Sponsors can move from protocol submission to First participant in significantly shorter timelines — without compromising on quality or compliance.
Why Porto, Why Astrum
Choosing Portugal for early-phase development offers Sponsors a combination of speed, safety, and global regulatory confidence that few European countries can match.
Regulatory advantage
Average approval times for Phase I studies.
With our Porto Phase I Unit, Sponsors gain the confidence of a trusted, globally recognized site combined with the speed, flexibility, and expertise of a pan-European CRO.
