With you through every phase
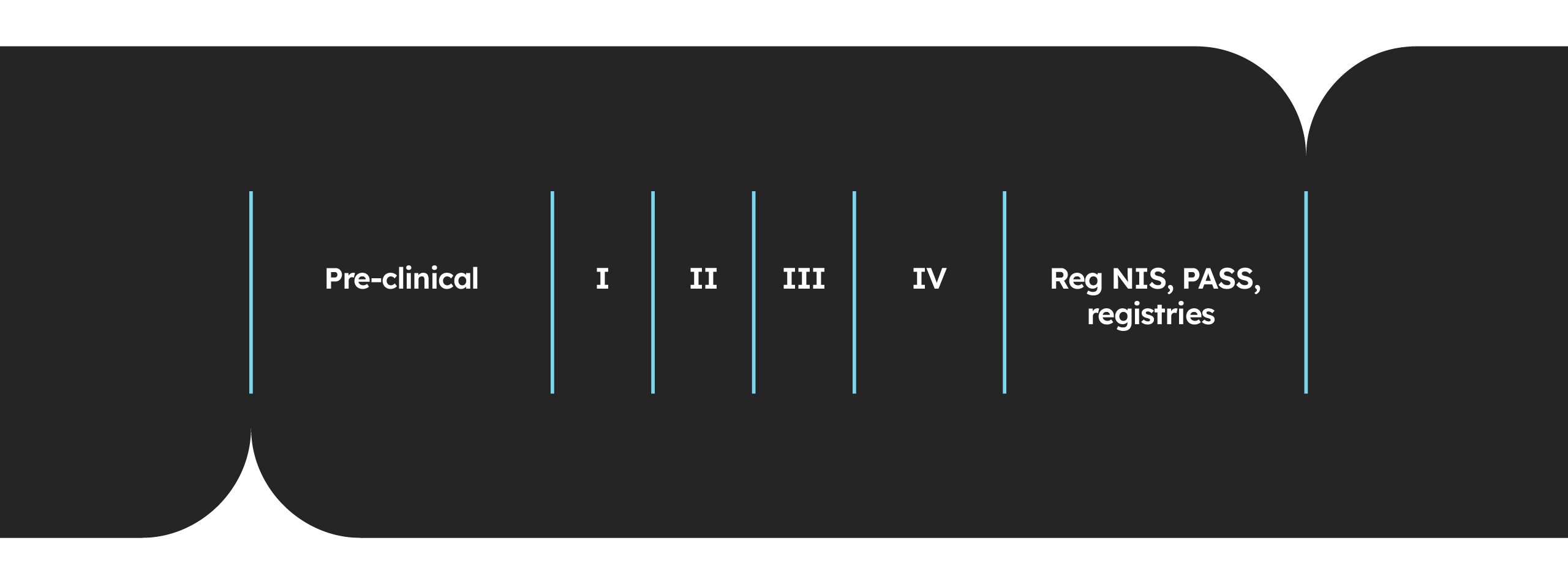
At Astrum we offer a full range of clinical development services spanning the entire drug development life cycle. Our client-focused approach coupled with our size means we can deliver end-to-end services or stand-alone solutions that are fully tailored to our client’s needs.
Our ability to adapt to the specific requirements of each project, maintaining focus on every detail, while always keeping sight of the bigger picture, ensures that we provide optimal solutions for our clients.
Services
Explore Astrum's wide array of clinical trial services below
Clinical Development & Scientific Advice
Ranging from first in human to post-marketing, Astrum’s clinical development expertise spans the entire development cycle, offering support every step of the way
Hospital-based Phase I unit
The Hospital-based Phase I unit in Porto, Portugal can quickly and safely establish your product’s safety, dose range, or bioequivalence/bioavailability
Pharmacokinetics & Pharmacodynamics
Your new drug’s absorption, distribution, metabolism, and excretion by the human body will be assessed by a highly-trained and experienced team
Biostatistics & Programming
Through state-of-the-art implementation, smart design, and statistical methodology, your study will be optimised for sample size, duration, and population
Project Management
Our team have extensive experience in project management and consultancy methodologies, offering our clients quality services that will deliver projects on time and within budget
Clinical Operations
Your trial will receive full support and expertise across Feasibility, Study Start-up, Site Management, Clinical Monitoring, and Trial Coordination and Administration
Quality Management
Our Quality Management System represents an unwavering commitment to excellence within the pharmaceutical, biotechnology, and clinical research sectors
Medical Monitoring
Acting as a point of reference for study team members and investigative sites, our Medical Monitors ensure the safety and integrity of the subjects throughout the trial
Medical Writing
We can flexibly support you from protocol to Clinical Study Report (CSR) writing. Across in-house clinical, regulatory, statistics, and medical colleagues, we’ll help achieve your study goals
Regulatory Affairs
We provide comprehensive regulatory services to help you navigate the ever-changing regulatory landscape and ensure efficient delivery of your clinical studies
Safety & Vigilance
We provide complete pharmacovigilance and medical device vigilance services from the early stage of development, through to the medicinal products and medical devices marketing
Data Management
Sponsors, investigators, and patients will receive comprehensive, end-to-end solutions for the collection and management of all clinical trial data
Additional Services
Our expertise extends to a wide range of services within multiple sectors. We pride ourselves on our ability to create and adapt solutions for a variety of clinical needs.
How can we help you?
Contact our expert team to discover how Astrum can bring value to your clinical development projects.